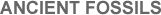
By what means do scientists know about the previous episodes of life on Earth? Never mind the precise details for now. How can we sketch even a broad outline of ancient events so far back in time? Part of the answer is that we’re guided by many clues preserved in the old rocks of our planet. Most of these hints are the remains of living organisms.
Life forms usually begin to decay as soon as they die. Once a means of gathering energy has ended, disorder sets in. Entropy takes its toll again, inevitably increasing according to the laws of thermodynamics. Dead organisms decompose quickly—even their bones eventually—their former proteins becoming rancid waste within a few days. This is the way in which all life forms, including humans, return to the planet the elements borrowed from it.
Some special environments can limit decay, including cold polar regions, high mountain tops, and deep ocean trenches. Both low temperatures and water burial serve to retard spoilage, so living systems that perish along a stream or ocean shore might end up sandwiched under layers of sand and sediment settling down through the water. Volcanic lava is another place where life forms can be buried yet preserved, in this case under mounds of ash. In time, the sedimentary deposits of sand or lava become hardened into rock, entombing the remains. Thus, though the fleshy parts of ancient organisms usually decay, their bony structure is sometimes preserved until later uncovered by natural causes (such as geological upheavals) or planned events (archaeological digs). These rare remains, or fossils, are the visible traces of dead organisms that once lived. Even boneless bacteria occasionally leave behind microfossils so small that a microscope is needed for analysis. Figure 6.3 depicts examples of these two ways to discover fossils.
| FIGURE 6.3 — Fossils can become exposed when (a) erosion wears away layers of sediment or (b) humans excavate the land. Studies of all the fossils thus far uncovered have enabled biologists to assemble a reasonably complete record of macroscopic life on Earth, but much of the microscopic microbial world remains to be discovered, catalogued, and interpreted. |
Exhaustive study of fossils thus far unearthed has enabled paleontologists to assemble a partial record of earlier life. These are the people who work at the interface of biology, chemistry, and geology, digging around amid dirt and rocks while seeking fossilized remnants of extinguished life. Using a variety of dating techniques, they can roughly determine when various organisms lived and sometimes how they died. More tellingly, the fossil record shows how, throughout eons of time, whole new life forms appeared while others disappeared. Some types of life survived for lengthy durations; others seem to have gone extinct nearly as soon as they arose.
As a rule of thumb, we can generally say that the oldest rocks embed only rare, simple life, whereas young rocks contain widespread, complex life. That’s not just a fanciful idea or subjective whim; it's an empirically demonstrated fact. The fossil record of biological specimens documents a clear and unmistakable trend over the ages. That trend, much as for galaxies, stars, and planets, is one of increasing complexity.
Oldest Life Forms All the fossils, taken together, chronicle a rich and varied natural history of life on Earth. The oldest fossils usually have a spherical, cellular structure resembling that of modern blue-green algae (now called cyanobacteria)—fuzzy, microbial scum found at the edge of today’s lakes, streams, and backyard swimming pools. Samples of ancient fossils are shown in Figure 6.4; these are older (~3.5 billion years) and less well preserved than those (~3 billion) shown earlier in Figure 5.2. Not overly complex, these earliest carbon-rich microfossils lack well-developed biological nuclei and display a plain, austere morphology only a few microns across; some of them are filamentary, as though the cells were loosely grouped on long chains. Yet, these life forms—microbes technically termed prokaryotes—of which the fossils are the remains, are controversial. Some of them could conceivably be minute carbon deposits formed by the action of scalding water on minerals trapped in the rock and not life at all. However and although recent contamination cannot be completely ruled out, the consensus interpretation among experts is that these findings, or others like them, are indeed old fossils of genuine life, possibly the remains of autotrophs that reproduced asexually by splitting in two. Some of the fossils do seem to show suggestive evidence of cells caught in the act of simple, binary division.
 |
FIGURE 6.4 – These fossils of ancient cells were photographed through a microscope and are dated to be ~3.5 billion years old. Some of them (top) seem grouped into filaments, although that interpretation is unclear; other fossils (middle frames) might be displaying replication, much as do modern unicells (bottom). All cellular structures here measure 5-10 microns across and have similar morphologies. (W. Schopf) |
Many of the best fossils of the most primitive, Archaean cells were discovered only within the past decade. Found embedded in South African and especially Western Australian rocks known to be ~3.6 billion years old, these oldest cells are presumed to have lived that long ago. In truth, paleobiologists have no way of dating the fossils themselves, nor are these very oldest remains fossils per se. Rather, they are fossil imprints of carbon deposits that were likely once bacteria, and radioactive techniques are useless for dating carbon older than ~40,000 years. Even so, it seems unlikely that latter-day algae could have gotten so deeply encased in such old rocks.
No fossils of the most ancient heterotrophs have ever been found. Tiny bits of elemental carbon (in the form of graphite specks) were recently discovered trapped in nearly 4-billion-year-old Akilia rocks of western Greenland, yet these oldest of all Earth rocks have been so heavily metamorphosed at high temperatures and pressures that most original organic information is lost, and the claim that life’s origin dates back that far is weak and equivocal. Molecules much smaller than cells can indeed seep into all but the densest of rocks, so there’s no good way of knowing if amino acids and nucleotide bases encased in rocks are genuine biosignatures as old as the rocks themselves. Even if paleontologists had methods to search for prebiotic organic matter, none would likely be found. The early heterotrophs probably devoured every bit of available organic matter, thus leaving no trace of the primordial soup anywhere on Earth; they simply ate all the evidence. Consequently, scientists are unable to estimate either the amount of time needed for the autotrophs to have overwhelmed the primitive heterotrophs, or for those heterotrophs to have appeared in the first place. As best we can tell thus far, life probably originated not more than a billion years after the formation of planet Earth. It could have conceivably emerged earlier, but how much earlier is currently only a guess.
Evidence of more recent, though still ancient, life has been unearthed in numerous places on our planet. The north side of Lake Superior in Ontario, for example, is especially rich in ancient fossils, and the rocks there are radioactively dated to be ~2 billion years old. No reputable scientist doubts this evidence for life. What’s more, these old life forms must have photosynthesized by some means or another, since chlorophyll is often found in their immediate vicinity.
Embedded within this old Canadian limestone are whole communities of cells called stromatolites—layered colonies of bacterial microbes in columns up to 0.5-meter tall, created when primitive autotrophs clustered together and became trapped in sediment that later hardened into rock. Figure 6.5a shows the fossilized remains of one of these early life forms. Living evidence for underwater stromatolites, closely similar in size and form to their ancient forebears and now providing the base of the ocean’s food chain, can be found today in shallow seas like Exuma Sound, Bahamas, and Shark Bay, Australia, where the water is too salty for plants and animals to graze on them—see Figure 6.5b.
Careful study of this 2-billion-year-old rock displays at least a dozen distinctly different types of cyanobacteria, all very much simpler than modern cells. Examined closely through a microscope, these old cells lacked well-developed biological nuclei and thus were still prokaryotic. And despite their obvious clustering, each of these ancient cells functioned on its own—as surely did even less well preserved stromatolites that are long dead, buried, and crushed into flattened mats within 3-billion-year-old Australian rock. All fossilized cells older than ~1 billion years appear unicellular, as each presumably failed to collaborate with other cells nearby. The reason might have been poor nutrition (that is, inadequate energy), ironically brought on by rising oxygen levels in the air.
| FIGURE 6.5— Blue-green algae cells sometimes cluster together to form underwater stromatolites. The fossilized ones shown in at left were trapped within rocky sediments ~2 billion years ago. Not all stromatolites are dead and fossilized; the club-shaped ones at right are alive and well in shallow water near Shark Bay, Australia. Both the sizes and forms of modern stromatolites resemble their fossilized ancestors, which represent the oldest known groups of prokaryotic unicells. (Canadian and Australian geological societies) |
Widespread evidence scattered across the globe makes clear that 1-2-billion-year-old rock formations often contain surprisingly well-preserved remains of autotrophs. Microfossils of at least 20 different types have been identified in one Australian outcrop alone, many of them again similar in structure to modern blue-green algae. More importantly, fossils toward the end of this period record the appearance of the first true organisms—clusters of interacting cells, the ancestors of modern plants and animals. In short, ~1 billion years ago, life had reached a whole new plateau. It was on the road toward increased organization.
Sex and Symbiosis Organization represents a distinct advance in complexity. By cellular organization, biologists generally mean that cells are communicating information, sharing resources, and working together as a team—we might say cooperating. Like inanimate stars and galaxies, the earlier microbial cells of stromatolites had clustered, but none of them displayed teamwork or communicated interactively. Biological organization ramps up the degree of ordered complexity considerably once collaboration begins among cells.
How did they do it? How did one or more cells learn to communicate, presumably for the common good? Apparently, some cells managed to evolve beyond the prokaryotic stage, thereby becoming eukaryotes—sexually reproducing life forms having genuine biological nuclei, including hereditary DNA molecules. Termed symbiosis to denote a mutually beneficial union of two dissimilar organisms, this process first jelled when an energy-poor, bacterial cell floating in the ocean swallowed another prokaryotic bacterium that had a talent for making the fuel-rich molecule, adenosine triphosphate, or ATP for short. The cell soon realized the benefits of an in-house energy factory and kept the bug as a permanent resident.
The oldest known fossils of unicellular eukaryotes (also called protists) date back nearly 2 billion years, and indirect chemical evidence implies that they might have existed well before even that. It’s probably not a coincidence that the first eukaryote—the common ancestor of all plants and animals—appeared at the time when free oxygen was on the rise in Earth’s atmosphere. Nearly all eukaryotes need oxygen to live, whereas most bacteria find it lethal. Even so, some bacteria managed to develop strategies to survive in an oxidizing atmosphere, mostly by taking refuge as parasites.
Today, deep inside each human (eukaryotic) cell, we do see hundreds of minute structures—mitochondria—that are widely thought to be descendants of that early (prokaryotic) bacterium. Colonies of bacteria have become indispensable passengers within the cells of every plant and animal. Living both on and in every person’s body are more bacteria than there are human beings on Earth; the millions of microbes in our gut aid digestion and produce essential vitamins. The bacteria are metabolic wizards that energize higher forms of life, their ATP enabling a variety of crucial functions such as muscle contraction needed for motion and protein construction needed to make more cells. These bacterial groups are the mitochondria that literally power most cellular activity by burning (via respiration) the food we eat. Cells acquire their energy by using a protein to ferry the ATP through the membrane of the mitochondria into the cells’ cytoplasm, a little like the nozzle at a gas-station pump that acts as an intermediary to transport fuel from the pump to a car.
Symbiosis was one of the great inventions of biological evolution—some say the most important event in the history of life (apart from its origin). At the least, symbiosis ensured bacteria’s future by becoming vital energy brokers for nucleated cells. Yet, ironically, it was also an advance that ended the microbes’ independent dominance of life on Earth.
How did sex overtake asexual reproduction some billion or more years ago, and how has it thrived ever since among many unicellular and nearly all multicellular organisms? Does sex have an advantage in the daily struggle for existence? All things being equal, asexual reproduction (involving only gene shuffling) ought to prevail on grounds of simplicity, the extra effort of having to find a mate limiting the success of sex (involving additionally gene mating and recombination)—yet sex reigns across much of the tree of life. Most researchers argue that sex greatly accelerates the rate of biological evolution by promoting genetic variety—and that alone might have been enough for Nature to embrace sex, once the unicellular eukaryotes figured out how to do it. But, with supporting evidence skimpy, perhaps it’s only wishful thinking that sex is good for the birds, bees, and us. Ideas abound, most having to do with either sex assembling beneficial mutations for the common good, or sex purging harmful mutations from the parental genome. The former would surely enhance the diversity of life, yet the latter would perform essential evolutionary editing lest deleterious mutations accumulate in individuals and in populations. Whichever, experiments are now underway in which biologists raise populations of organisms ranging from water fleas to mud worms, thereby testing lineages’ fitness despite imposed mutations. Thus far, the results are mixed, quite possibly because both mechanisms are at work—sex collects good mutations and rejects bad ones—another case of “gray compromise” so often orchestrated by Nature in the wild.
By ~1 billion years ago, unicellular life had already existed on Earth for >2 billion years. Its basic cells had become ~10 times crossectionally larger (thus ~1000 times more voluminous), vastly more sophisticated, and perhaps more diverse functionally. Furthermore, fossilized cells of this age show clear and widespread evidence for eukaryotes, which have much different structures from single-cell bacteria. More advanced life was emerging from simple life, albeit coexisting with it all the while. Equally important, some of the 1-billion-year-old organisms had discovered ways to enhance their survivability by working together as groups; they had become multicells that did collaborate with one another.
But that’s all there was ~1 billion years ago. Primitive oceanic life flourished, though not much else. The fossil record shows no evidence that plants yet adorned Earth’s landscape. No animals were crawling, swimming, or flying near the surface. And certainly, by no means were men and women yet even on the evolutionary horizon.