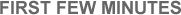
To appreciate the earliest PARTICLE EPOCH of the Universe, we must be willing to think deeply about times long, long ago. We must strive to imagine what it was like well before the Earth and Sun emerged, even before any planet or star existed. Some people have trouble mentally visualizing such truly ancient times. Fortunately, a trick can help us comprehend the earliest moments of the Universe when particle evolution dominated.
A Symmetry Argument Physicists are mainly charged with the application of the laws of Nature to the present state of something in order to predict its future. Although, in recent years, a renewed respect for the role of chance has somewhat diminished our ability to predict outcomes in the old, mechanistic, Newtonian sense, we still like to try our hand at predicting general trends, if not the details. In the case of the whole Universe, that “something” is literally all things—nothing in particular, just everything in general. Hence, if we find it hard to mentally reverse time to appreciate the earliest epoch of the Universe, we can instead take advantage of the natural symmetry of a model Universe that will eventually contract, and thereby predict the physical events destined to occur as a closed Universe nears its final phase of total collapse (see Figure 1.16). This procedure is valid only because the mathematics describing contraction are a mirror image of those for expansion. In other words, the events that will occur just prior to the end of a contracting Universe mimic those that already happened just after the start of an expanding Universe. Not that time ever does reverse, as best we know. Rather, we can use some of the symmetry built into the laws of physics to estimate the final events of such a hypothetically closed Universe, thus gaining some inkling of the initial events ~14 billion years ago.
| FIGURE 1.16 — Schematic diagram of the expansion and contraction of a closed Universe. The shaded parts show how the physical conditions are roughly the same near the beginning and end of this model Universe. |
Even if the real Universe is not closed in this way and will never collapse to a singularity, astrophysicists employ closed models in order to understand theoretically some of the highlights of the earliest epoch of either a closed or an open evolutionary Universe. It’s an example of how we can use symmetry and scaling arguments—to scale models up, or scale them down, in this case to scale them back in time—in order to recreate mentally places and times we could never actually visit physically.
“Numerical experiments” are used to compute the Universe models. These are essentially number-crunching exercises, utilizing mathematical knowledge of physical laws and sophisticated software running on powerful computers. The resulting simulations are ponderous and computationally intensive, incorporating much of what we know about the bulk features of the Universe—again, largely generalities minus the messy details. The objective is to determine the average density and average temperature for the whole Universe at any moment in time. The input numbers, such as mass, energy, and expansion rate, can be varied as the computer routines are run again and again, the idea being to match the resulting state of the model Universe with that of the currently observed Universe. In this way, the range of input values can be progressively narrowed, thereby converging on a description of the Universe that reasonably mimics reality.
Typical results of these computer models are collected in Table 1-1, plotted in Figure 1.17, and sketched in Figure 1.18 for several key periods throughout universal history. These lists include later intervals of time, such as the galactic period and the stellar period, whose subjects are best treated later in this Web site.
 |
FIGURE 1.17 — Plots of the average temperature and average density throughout the history of the Universe. Numerical values for these plots are taken from Table 1-1. |
| FIGURE 1.18 – Artist’s conception of the main events during 6 major periods of the Universe corresponding to Table 1-1. The sketches are idealized, yet for each period capture the essence of changing temperatures, densities, and structures. (Lola Chaisson) |
Computer Modeling Most computer models suggest that in the beginning, there was chaos! But, frankly, we are sometimes unsure if the chaos was in the Universe or is now in our computer codes. Again, the problem is the so-called singularity at the moment of the big bang itself—a decidedly odd state about which mathematicians are currently perplexed. It’s hard to imagine that science will ever be able to prove what happened at the exact moment of the bang—precisely zero time. That’s why big-bang cosmology, contrary to popular belief, is not a theory of the big bang per se. Rather, it’s a cognitive map, or worldview, that aspires to explain events in the aftermath of the big bang.
Many theorists contend that the physical conditions can be approximated for extremely short times after the bang, well less than the first second of existence. For example, most models specify that a Universe younger than a trillionth of a trillionth of a second (i.e., 10-24 second) would have had an average density greater than 1048 g/cm3, and an average temperature greater than 1021 K. By way of comparison, the average densities of water and lead are 1 and 10 g/cm3, respectively, and of atomic nuclei ~1012 g/cm3. Also, the present average density of all material objects in the Universe is roughly a million trillion trillion times less than that of water (or about 10-30 g/cm3); this is the average density of everything—galaxies, stars, planets and life forms, as well as mostly empty space. Likewise, water freezes at 273 K and boils at 373 K, while the average temperature at the surface of an ordinary star is several thousand Kelvins. The present temperature of everything in the Universe, again on average, is only a few degrees above “absolute zero,” ~3 K.
As for the time just noted, it’s nearly impossible to appreciate such youth; 10-24 second is the duration needed for light to cross a proton—the nucleus of the smallest atom. Such minute fractions of time—much quicker than a flash, literally—are as incomprehensible as the huge densities and extreme temperatures characterizing the early Universe. Yet these are the conditions specified by the laws of physics as a contracting Universe inexorably speeds toward its demise. They are thus, through the above symmetry arguments, the conditions thought to have prevailed in those violent moments shortly after the birth of the Universe.
The composition of the Universe at such extraordinarily early times is also hardly describable. Vast amounts of energy must have existed in the form of pure radiation, along with exotic elementary particles of many types, but beyond that science can currently only speculate. The dominant action at the start of the PARTICLE EPOCH was hardly imaginable. Undaunted, we shall return below to take a stab at it, but first we pause to remind ourselves of some relevant things we do know and understand well.
Basic Particles Here, we sidetrack for a brief review of the fundamental makeup of matter and a short note about the basic forces that govern it. By this we mean normal, or baryonic, matter, putting aside dark matter for now, given that there are so few clues about what it really is.
Exploration of the basic nature of ordinary matter is not new. At least as far back as ancient Greece, attempts were made to discern the composition of all things. Although clearly great thinkers, the Greek philosophers were deeply in error; they presumed that thinking about Nature was better than looking at it. Still, their ideas prevailed for more than 2,000 years, culminating in the witchcraft and magic that befuddled the efforts of medieval astrologers and dark-age alchemists.
Only with the rise of logical, deductive reasoning during Renaissance times, and especially its heavy reliance on testing, did the technique of “experimental philosophy” become fashionable. At last, a proper balance between thinking and looking was achieved. The technique is simple: Thoughts (theoretical work) are to be taken seriously only if confirmed by tests (experimental work). Modern science thereby emerged and with it the “scientific method.”
Not that the scientific method is entirely objective, as confessed in the SITE SUMMARY of this Web site. Science is practiced by human beings, and scientists are no different from others who have subjective emotions and personal biases. Yet over the course of time, criticism, and debate, scientific issues eventually gain a measure of objectivity. By incessantly demanding repeated tests and proven facts, the scientific community gradually damps the subjectivity of individuals and arrives at a more objective view among a community of critical thinkers. Skepticism and doubt are essential features of the modern scientific method.
In one of the greatest triumphs of the scientific method to date, physicists of a century ago were able to prove that atoms are not the most basic entities of Nature. All atoms of every different kind—that is, all elements—are made of negatively charged electrons whirling around positively charged nuclei. Each neutral atom has equal numbers of electrons and protons, as well as a similar number of neutrons. The protons and neutrons contain virtually all the mass of any atom, and together they comprise the atom’s nucleus—so compact relative to the size of the larger atom as to resemble a grain of sand floating alone amid a sphere the size of a football stadium.
For the first half of the 20th century, electrons, protons, and neutrons, along with photons of radiation, were considered the very essence of material substance. However, during the second half of the century, physicists also discovered a bewildering array of additional elementary particles. These newer particles are not likely any more “elementary” or basic than the better-known protons and electrons. Rather, each one seems to play its own role in the subatomic realm far from everyday familiarity. And that role is not always clear, as none of them can be seen directly; they can only be inferred when interacting with other matter while passing through laboratory equipment.
More than 200 elementary particles are currently known—which makes one wonder just how elementary, or fundamental, they really are. Some behave like lightweight electrons, whereas others resemble heavyweight protons. Still others display bizarre properties not yet understood. Many particles exist for only fleeting moments during fierce collisions induced in high-energy accelerators—vast underground laboratories where, typically, electrons and protons are boosted to velocities near the speed of light and then slammed together violently. The largest and most powerful of these machines are the Conseil Europeen pour la Recherche Nucleaire (CERN), which houses a complex circular laboratory 100 meters underground and 27 kilometers in circumference across the French-Swiss border near Geneva, and the Fermi Laboratory that spans an area nearly as large beneath a Chicago suburb. The new particles literally materialize from the energy of the collisions; no magic is involved as this is a well-understood physical process. Usually, after a microsecond or so, the particles change back into energy, but not before leaving behind momentary traces on the accelerators’ detectors.
The history of efforts to decipher the building blocks of Nature is full of false claims. Each time researchers thought they had discovered a truly basic component of matter, they have been proved wrong. With molecules now known to be made of atoms, and atoms in turn made of elementary particles, other questions naturally come to mind: How elementary are the new particles seen in the debris of accelerator collisions? Are these particles perhaps made of even more fundamental subparticles that have some identity or existence of their own? Current theory and some data do suggest another layer of fundamentality.
The prevailing view in today's scientific community is that protons and neutrons, among a whole menagerie of elementary particles (called “hadrons”) with sizes ~10-13 cm, are made of units called quarks, and together they comprise more than 99% of normal, baryonic mass in the Universe; the rest is made mostly of dimensionless electrons, which are not dividable into quarks or apparently anything else. Quarks (which derive their name from a meaningless word coined by the novelist James Joyce in his book, Finnegan’s Wake) are minute particles having only a fraction of the electric charge carried by a proton. For example, a proton consists of two “up” quarks (each with 2/3 charge) and one “down” quark (having a -1/3 charge); a neutron has one up and two down quarks. Over the past few decades, an intricately detailed yet remarkably successful theory, called quantum chromodynamics (or QCD, for short), has been refined around 6 quarks having the metaphorical names of up, down, top, bottom, strange, and charm, each variously bound by yet another elementary entity, the gluon. Despite its inherently fuzzy (quantum) nature and oft-intractable equations, this mathematically elegant theory, aspects of which have generated Nobel Prizes for more than 20 physicists, underlies many popular products in today’s technological world, including televisions, lasers, cell phones, among a whole industry of electronic devices built on computer chips.
Originally, when the idea of quarks was first proposed several decades ago, they were mostly judged to be no more than a mathematical convenience—a mental bookkeeping system for describing quantum interactions yet not real objects that could be studied tangibly. Nowadays, accelerator experiments clearly demonstrate the physical existence of the 6 different kinds of quarks, mainly by observing the way fast-moving electrons deflect when fired at protons. These events involve violent, head-on collisions, a little akin to a hypothetical attempt to understand the makeup of a clock by smashing two of them together at high speed. Traces of all 6 quarks have now been found in the accelerator debris and together they form the essence of the standard model of particle physics—a popular consensus of submicroscopic phenomena, bolstered by accelerator experiments and the quantum theory of particles and forces. Yet no compelling reasons exist to prohibit Nature from having more such particles, indeed the 6 quarks are thought to have 6 partners (called “leptons”) of which the electron is one. All of which suggests a conundrum: The very proliferation of quarks and their relatives threatens to topple the central idea that we have reached a truly fundamental realm of matter.
On paper (that is, in highly theoretical terms) and on very basic scales (that is, much smaller than even that of quarks)—thus well removed from anything testable currently, if ever—physicists are actively investigating nearly intractable mathematical models that seek to interpret particles, not as minute points, but as “strings.” This approach, known as string theory, envisions matter on scales as small as 10-33 cm—some 20 orders of magnitude smaller than a proton—as modes of vibration among ultra-submicroscopic items that, if we could see them, would resemble strings and loops throbbing in well more than four dimensions of spacetime. As complex as it sounds and as remote as it is from everyday practicality, string theory is considered “beautiful” and “elegant” by the experts pursuing it, many of whom feel that it offers the best hope to unify all the known forces of Nature. We shall return to reconsider strings later in this PARTICLE EPOCH.
Basic Forces As best we can tell, the behavior of normal matter on all scales—from elementary particles to clusters of galaxies—is ruled by just a few basic forces. Forces and the fields and energies they engender are the root cause of changes everywhere; they are fundamental to everything in the cosmos. In a sense, the search to understand the nature of the Universe is synonymous with the quest to understand the nature of these forces. Forces, fields, and energies are among the essential keys needed to unlock some of the most concealed secrets of the Universe.
The gravitational force is perhaps the best known force. Gravity binds galaxies, stars, and planets, and it also of course holds us on Earth. Like other forces, its strength decreases with distance from any object; in fact, it decreases as the square of the distance, and is said to obey the “inverse-square law.” However, that’s only half of the law of gravity, as its strength is also proportional to mass. Thus, gravity is terribly weak near, for example, a puny atom, but enormously powerful near a huge galaxy. In fact, although gravity is by far the weakest of all of Nature’s known forces, its effect can accumulate impressively over large volumes of space that contain mass. Nor can anything cancel the attractive pull of gravity; there is no such thing as antigravity that repels objects—at least not for normal matter. Even the peculiar stuff known as antimatter (discussed shortly) has gravity, not antigravity. Consequently, the gravitational forces of all objects—including our own bodies—extend to the outer limits of the Universe, hence the reason why gravity is known as a “long-range” force. To be sure, on scales larger than Earth, gravity is the dominant force in the Universe.
The electromagnetic force is another of Nature’s basic agents. Any particle having a net electric charge, like an atom’s electron and proton, exerts an electromagnetic force. This force acts as the cement for most ordinary materials, including virtually everything in our homes, such as tables, chairs, books, even the kitchen sink. Because the electromagnetic force also binds the atoms within all life forms, some biologists call it the “life force”—which, unfortunately, leads some to think that life is governed by some special “vitalism,” which is wrong. Like gravity, the strength of the electromagnetic force decreases with distance according to the same inverse-square law. But unlike gravity, it can repel (between like charges) as well as attract (between opposite charges). Such forces can then sometimes cancel one another, as when similar numbers of positive and negative charges neutralize the electromagnetic force, thereby diminishing its influence. For example, although a human body is made of ~1029 charged particles, it comprises almost equal mixtures of positive and negative charges; our bodies therefore exert hardly any net electromagnetic force. Overall, electromagnetism is much stronger than gravity on microscopic scales and smaller, but is much less influential on macroscopic scales where gravity rules.
A third fundamental force is termed the weak force, as its effective range is less than the size of an atomic nucleus and its influence on matter much more subtle than any of the other forces. We shall not encounter it much in the course of describing cosmic evolution, except to note that the weak force helps to change one kind of elementary particle into another (such as the arcane neutrino particles released during nuclear reactions at the Sun’s core). The weak force also governs the emission of radiation from radioactive atoms, which are useful in establishing dates that, in turn, reveal the tempo of cosmic evolution. Most scientists now agree that the weak force is not really a separate force at all; rather, it’s probably another form of the electromagnetic force acting under peculiar circumstances. As such, we now often speak of the “electroweak force,” an idea to which we shall return in the next section.
A stronger force than any of these is the nuclear force, mediated by the gluon particle that holds the quarks together. It glues—hence its name—protons and neutrons within atomic nuclei and, in effect, serves as the source of energy in the Sun and stars. Like the weak force, yet unlike the forces of gravity and electromagnetism, the nuclear force operates only at very close range; it’s useless when matter is separated by more than ~10-12 cm. But within this range, as for all atomic nuclei, it binds particles with enormous strength—stronger, in fact, than any other force known. Numerically, and in absolute terms independent of their most potent ranges, the nuclear force is 137 times stronger than the electromagnetic force, 100,000 times stronger than the weak force, and 1039 times stronger than gravity. Ironically and despite its extraordinary weakness, gravity is the only force that affects all things at all times on all scales.
And, then, there is dark energy, as noted in the earlier cosmology section of this PARTICLE EPOCH, which implies another, perhaps wholly new fifth force about which science is thus far truly perplexed.
The Early Universe By most accounts, the Universe originated with the expansion of an unbelievably hot and dense “something”—hotter than the tens of millions of Kelvins in the cores of most stars, denser than the trillions of grams per cubic centimeter in the nucleus of any atom. Precisely what that state was, we cannot say for sure. And why it “exploded,” we really don’t know. At best, science contends that in the beginning a singularity released an outward burst of pure, radiant energy. Why the Universe suddenly began expanding more than 10 billion years ago is a most intractable query—so formidable that scientists are currently unaware even how to formulate a meaningful question about it.
In the broadest sense, there are what questions, how questions, and why questions. Using astronomical telescopes and biological microscopes, among an arsenal of other experimental gear, researchers have employed the reductionist approach that has served science so well since Renaissance times to unravel both the macroscopic and microscopic nature of matter—namely, to tally fairly well what exists in the Universe—from atoms to galaxies, and from cells to brains. Not that our inventory is complete by any means, for the nature of dark matter (let alone dark energy) remain unsolved. Yet, armed with a rather detailed inventory of what astronomers call “normal” matter—which comprises all that we perceive directly in the Universe—we are able to address the origin and evolution of that matter, in other words, how it got there initially and how it has changed ever since.
To inquire about the nature of the very beginning, however, requires us to address why questions, the most fundamental of all being, Why is there a Universe at all? To be honest, scientists don’t know how to tackle why questions. These are outside the present fabric of modern science and probably always will be. In other words, when a ball is put in motion and Newton tells us that the ball “will remain in motion unless or until it is acted upon by some external force,” we have no understanding why it does that. We do know what such balls do and also how they do it, but why we have no clue. No known procedure—not even the vaunted scientific method—enables us to investigate why, in the deepest sense of that word, the laws of physics and biology are as they are. We shall probably never know the answer. Nor do we know, or likely have any prospect of ever really knowing, why there is a Universe—or what might have preceded its origin.
The basic problem in attempting to discover the nature of what, if anything, existed prior to the very start of the Universe is simple: There are no data. None whatsoever. Sure, some people have hypotheses, but these are not based upon data. Any such notions are in every case contingent largely on thoughts or beliefs, and while noble and comforting to many in society they cannot be considered science. The methods of inquiry used by scientists and those used by philosophers and theologians are as different as oil and water; they just don’t mix. To be crass about it, if an idea is experimentally or observationally testable, then it qualifies as science; if it’s not, then it’s something else.
This is not a criticism of humans who wonder about the start of the Universe, or even about what might have come before it. Long ago, Augustine related a popular 5th-century idea that before creating heaven and Earth, God made hell for those who worry about such issues. Augustine himself was more likely correct in thinking that the Universe was made with time, not in time; that's also today’s prevailing view among most scientists, namely that time started at the moment of the big bang and that before that nothing existed, not even time. Even so, a minority of researchers occasionally ponder how to devise experiments to gather pre-universal data. However, as things stand now, queries about the nature of whatever existed before the bang amount to inquiries, less about the origin of the Universe and more about the origin of the origin. Not to be overly critical, what came before the big bang might well be a meaningless puzzle—like the popular Medieval exercise of counting angels balanced on the head of a pin—since at the beginning of the Universe, matter, energy, space and time probably all came into being. Resembling the “here-there-be-dragons” school of ancient cartography, the concept of time before the big bang is quite possibly nonsense. To most cosmologists, asking what happened prior to the big bang is akin to asking what lies north of the North Pole!
In what follows, we necessarily confine our discussion to events extant since the start of the Universe, based on our empirical knowledge of its existence during the past 14 billion years, and regardless of why the Universe did originate. Indeed, cosmic evolution constitutes a broad synthesis of the whats and hows, and not at all of the whys.
Hadrons and Leptons Within a microsecond (10-6 s) of its being, the fiery Universe was flooded with energy throughout every available niche. It was also peppered with a whole mélange of subatomic particles of matter, whizzing this way and that amidst unfathomably intense heat and light. Whence did these particles come? From radiation, pure and simple. The particles “materialized”—a creation of sorts—as matter was literally fashioned from the energy of the primeval bang. Neither magic nor mysticism prevailed, just a well-known and oft-studied fact that elementary building blocks of matter often emerge from clashes among packets of energetic radiation. As shown in Figure 1.19, the interchangeability of matter and energy is proved daily in the underground bowels of particle accelerators around the world, the two obeying that most famous of all formulas noted earlier in this PARTICLE EPOCH: E = mc2.
| FIGURE 1.19 — Tracks of elementary particles observed in high-energy accelerators often display particle-antiparticle creation. Here, a gamma-ray photon arrives from the right, suddenly yielding its energy to produce an electron-positron pair. The pairs of particles curve in opposite directions in the detector’s magnetic field because of their opposite electric charges. (CERN) |
Foremost among the particles made well within the first second of existence were the quarks and their gluon comrades. A quark-gluon plasma, colloquially known as “quark soup,” prevailed in the Universe just prior to the natural emergence of protons, neutrons, and other heavy elementary particles that are built of quarks. (Plasma, the “fourth state of matter” after solids, liquids, and gases, is comprised exclusively of charged particles, normally protons and electrons not bound within atoms.) As bizarre as this stuff seems, quark soup was actually verified—that is, a whole new state of matter created—in one of the first notable accelerator experiments of the 21st century. As shown in Figure 1.20, physicists did it by slamming together two gold nuclei at 99% of the speed of light and then examining the thousands of particles that sprayed out from the ensuing meltdown. The superenergetic result was a seething concoction of free-roaming quarks and gluons—a miniature fireball of sorts—momentarily produced and controlled in the laboratory.
| FIGURE 1.20 — By violently smashing the building blocks of matter and examining debris from the ensuing collisions, physicists infer knowledge about the basic forces and the structure of matter at very small scales and very high energies. Here, myriad particles emanate from the site of two gold nuclei having collided, the result being “quark soup” which approximates the incredibly hot and dense conditions that likely prevailed within the first second of the Universe’s existence. (Brookhaven National Laboratory) |
Soon thereafter, yet still only about a microsecond after the big bang, the massive, strongly interacting elementary particles such as protons and neutrons—those collectively called “hadrons”—became the most abundant types of matter. Such particles must have then existed as free unbound entities, given the inferno prevalent in the Universe well within its first second of existence. It was just too hot for these particles to have assembled into anything more ordered. Hadrons surely collided and interacted with one another as well as with other types of elementary particles, for the density then was also extremely high. Accordingly, the dominant action during this hadron period was the inception and then self-annihilation of hadrons into radiation, which further fueled the brilliant fireball. Lacking a good understanding of elementary particles at the highest energies, physicists have only partial knowledge about this puzzling period in cosmic history.
One fact we do know is that energy reigned supreme, vaporizing all but the smallest chunks of matter. Protons, neutrons, and electrons, as well as a veritable zoo of other submicroscopic particles were unable to cluster into more complex structures. No stars or planets existed at the time. Not even any atoms were tolerated. The environment was too energized, the Universe still too chaotic—the clear and frenzied aftermath of the biggest of all cosmic “bombs.”
The basic stuff of the Universe continued to fly apart rapidly, cooling and thinning all the while. About a millisecond (~10-3 s) after the bang, the superhot and superdense conditions suitable for hadron creation had ceased, allowing a whole new class of particles such as electrons and neutrinos to come forth and dominate. Thus began another process of materialization whereby lightweight, weakly interacting particles—those called “leptons”—were fashioned from energy under an average density of 1010 g/cm3 and a temperature of ~1010 K. These physical conditions were still excessive by any earthly standards, but they had moderated greatly compared to the hugely denser and intensely hotter values present a fraction of a second earlier. For once the Universe began expanding, it did so extraordinarily rapidly, unhesitatingly dispersing its heat and its contents. By the time the first second had elapsed, leptons were being quickly made from radiation and many just as quickly destroyed back into radiation, much as had the hadrons earlier. In a kind of equilibrium between creation and destruction of subatomic particles, this cosmic fireball was still fueled during this lepton period with harsh radiation, such as x rays and gamma rays, as well as with (what we would now call) blinding light.
The density of radiation greatly exceeded the density of matter throughout these first few minutes. Not only did the photons of radiation far outnumber the particles of matter, but also most of the energy in the Universe was in the form of radiation, not matter. As soon as the elementary particles tried to combine into atoms, fierce radiation destroyed them. Structure, organization, and complexity did not yet exist; information content was minimal. Radiation was simply overwhelming, and for this reason much of the PARTICLE EPOCH is often called the Radiation Era. Whatever matter managed to exist at the time did so as a thin precipitate suspended in a glowing “fog” of dense, brilliant radiation.
Antimatter An atom of ordinary matter is an invisible, submicroscopic entity made of a positively charged heavyweight nucleus, usually several protons and neutrons, surrounded by one or more negatively charged lightweight electrons. All atoms found on Earth maintain this common structure—the essence of normal, baryonic matter. Furthermore, radiation received from extraterrestrial objects, near and far, is consistent with this same basic structure for all atoms everywhere.
Theorists nonetheless ponder the possible existence of other kinds of atoms—not just additional elements yet undiscovered, but atoms built differently from the ones we know on Earth. How is it, for instance, that heavy nuclei always have a positive charge, relegating the negative charge to only the lightweight electrons? Some argue that the Universe would be more philosophically pleasing if its basic building blocks had more symmetry in their charge and mass. In other words, perhaps the Universe is also endowed with atoms made of negatively charged nuclei around which orbit positively charged particles.
Experimentalists did in fact discover, around the mid-20th century, lightweight, electronlike particles having a positive charge. These so-called antimatter particles are identical to ordinary matter particles in every way except charge. A particle called a positron, for example, has all the properties of an electron, except that its charge is positive. These same experiments also proved that when a matter particle and its antimatter opposite collide, the result is mutual matter-antimatter annihilation, an explosion that releases pure energy of the lethal gamma-ray variety.
The reverse phenomenon can occur as well. Provided the temperature is extraordinarily high (in the range of billions of kelvins), collisions among packets of gamma radiation can yield pairs of elementary particles, for instance, a matter electron and an antimatter positron. This sort of materialization (or “pair production”) of matter and antimatter from energy still obeys the fundamental laws of physics; in this case, once again in accord with the formula, E = mc2.
These and other kinds of conversion from energy to mass are precisely what theoretical models suggest happened in the earliest moments of the Universe. Yet we don’t observe much antimatter around us now. Earth, the other planets, and the Sun all appear to be made of ordinary matter. Exceptions include some particles produced in nuclear reactions known to be churning away inside stars; a small fraction of the baffling cosmic rays showering us each day; and minute fragments created during elementary-particle collisions in nuclear laboratories on Earth. Still, virtually all the mass in the Solar System seems to be of the matter variety with little trace of naturally occurring antimatter. If matter and antimatter were created in equal amounts from primordial energy in the early Universe, then where has all the antimatter gone?
Note that antimatter does not imply antigravity. Particles of antimatter gravitationally attract one another just as do two or more particles of matter. The only property distinguishing matter from antimatter is charge; the mass of every matter particle is identical to that of its antimatter opposite, hence gravity invariably pulls while never pushing. Apart from the mysterious “dark energy,” discussed earlier in this PARTICLE EPOCH but not yet found, no such “antigravity” effect is known anywhere in the Universe.
Nothing, in principle, prohibits elementary particles of antimatter from combining into large clumps. Antihydrogen, antioxygen, anticarbon, and numerous other antiatoms could conceivably form antiplanets, antistars, antigalaxies, and presumably antilife. The fact that we are unaware of such antimatter objects doesn’t preclude their existence. Since atoms of antimatter emit and absorb precisely the same type of photons as do atoms of ordinary matter, astronomers have no way to determine if, for example, a distant star is made of matter or antimatter. Physicists like to say that a photon is identical to its own antiphoton. Radiation emitted by a clump of antimatter would equal that from a clump of matter; photons and antiphotons have no known differences. Accordingly, the nearby Alpha Centauri star system or the Andromeda Galaxy, for example, could be composed of antimatter—but it’s doubtful.
Despite the fact that our Solar System is composed mainly if not totally of matter, large pockets of antimatter may well exist elsewhere in the Universe. Provided clusters of matter remain separated from those of antimatter, then the two can coexist. As to where the primeval antimatter might now be, we can only conjecture that it’s wrapped up in large, distinct assemblages far outside the Solar System. Should similar matter and antimatter objects venture too closely together, however, they would mutually annihilate. Consequently, if our civilization ever attains an ability to travel beyond our Solar System, it will be important to dispatch automated probes before humans visit alien worlds. Should such an unmanned spacecraft suddenly evaporate in a burst of gamma radiation, we would be wise to visit elsewhere.
That said, scientists presently have no experimental evidence for macroscopic structures of antimatter beyond Earth. They are only theoretically possible, if not probable, on the basis of symmetry arguments: The simplest cosmological models imply that equal quantities of matter and antimatter should have been created from energy in the PARTICLE EPOCH. We are not done with this dilemma.